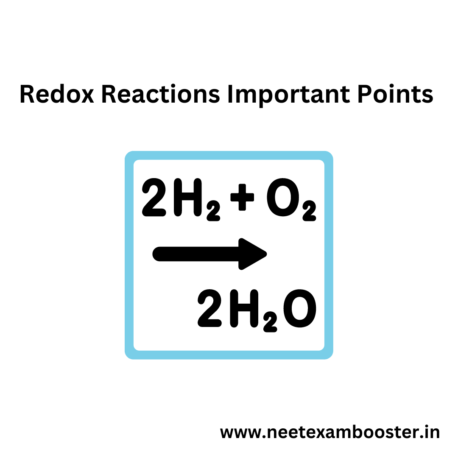
Redox reactions important points : Redox reactions involves transfer of electrons between reactants, where one substance is oxidized and the other is reduced.
Oxidation is loss of electrons, while reduction is gain of electrons. Redox reactions can be balanced using half-reaction method, and they are used in batteries, fuel cells, biological systems, chemical synthesis, and industry.
Redox reactions are identified by a change in oxidation state, and they can be used to produce the energy or create new compounds.
Redox reactions important points, Redox reactions important points, Redox reactions important points, Redox reactions important points, Redox reactions important points, Redox reactions important points, Redox reactions important points, Redox reactions important points

NCERT Chemistry Clas 11 Chapter 8 – Redox reactions important points 25 Important Points
There are 25 important points on Redox reactions –
- Redox reactions involves the transfer of electrons between the reactants.
- The term “redox” comes from words “reduction” and “oxidation”.
- Reduction is the gain of the electrons by a substance, while oxidation is the loss of electrons.
- Redox reactions always occur in pairs: one substance gets oxidized while the other gets reduced.
- The substance that is oxidized is called as the reducing agent, while the substance that is reduced is called as the oxidizing agent.
- The reducing agent loses the electrons and becomes more positive, while oxidizing agent gains the electrons and becomes more negative.
- Redox reactions can be identified by presence of a change in oxidation state.
- Oxidation state refers to hypothetical charge that an atom would have if all the electrons in a compound were equally shared.
- Element in its elemental form has an oxidation state of 0.
- The sum of oxidation states in a neutral compound is 0.
- The sum of oxidation states in an ion is equal to the ion’s charge.
- The more electronegative the element in a compound is assigned a negative oxidation state.
- Redox reactions can be balanced using half-reaction method.
- In half-reaction method, the oxidation and reduction reactions are written separately.
- Electrons are added to oxidation half-reaction to balance the charges, while electrons are removed from the reduction half-reaction.
- The balanced half-reactions are then combined to form overall balanced redox reaction.
- Redox reactions can be used to produce the electricity in batteries.
- In a battery, oxidation occurs at anode, while reduction occurs at cathode.
- Redox reactions can also be used to generate the energy in fuel cells.
- In a fuel cell, hydrogen is oxidized at anode, while oxygen is reduced at the cathode.
- Redox reactions are important in the biological systems, as they are involved in the cellular respiration and photosynthesis.
- Cellular respiration involves oxidation of glucose to produce ATP.
- Photosynthesis involves reduction of carbon dioxide to produce glucose.
- Redox reactions can also be used in the chemical synthesis to create new compounds.
- Redox reactions are widely used in the industry, for example, in production of metals and in the manufacture of chemicals such as chlorine and hydrogen peroxide.
Some Important Questions From Biology Class 11
| Chapter Name | Quiz Link |
| The Living World | Play Now |
| Biological Classification | Play Now |
| Plant Kingdom | Play Now |
| Animal Kingdom | Play Now |
| Morphology of flowering plants | Play Now |
| Anatomy of flowering plants | Play Now |
| Cell: the unit of life | Play Now |
| Biomolecules | Play Now |
| Cell Cycle and cell division | Play Now |
| Transport in Plants | Play Now |
| Structural organisation in Animals | Play Now |
| Mineral nutrition | Play Now |
| Photosynthesis in higher plants | Play Now |
| Respiration in plants | Play Now |
| Plant Growth and development | Play Now |
| Digestion and Absorption | Play Now |
| Breathing and Exchange of Gases | Play Now |
| Body fluids and circulation | Play Now |
| Excretory products and their elimination | Play Now |
| Locomotion and Movement | Play Now |
| Neural Control and Coordination | Play Now |
| Chemical Coordination and Integration | Play Now |
Some Important Questions From Biology Class 12
| Chapter Name | Quiz Link |
| Reproduction in organism | Play Now |
| Sexual reproduction in flowering plant | Play Now |
| Human reproduction | Play Now |
| Reproductive health | Play Now |
| Principles of inheritance and variation | Play Now |
| Molecular basis of inheritance | Play Now |
| Evolution | Play Now |
| Human health and disease | Play Now |
| Strategies for enhancement in food product | Play Now |
| Microbes in human welfare | Play Now |
| Biotechnology principles and processes | Play Now |
| Biotechnology and its application | Play Now |
| Organism and population | Play Now |
| Ecosystem | Play Now |
| Biodiversity and its conservation | Play Now |
| Environment issue | Play Now |



 155 out of 200 questions were directly asked from these notes in NEET 2024
155 out of 200 questions were directly asked from these notes in NEET 2024
