General principles and processes of isolation of elements important points – The general principles and processes of isolation of elements in chemistry involve various steps, including mining, concentration, extraction, and refining. Metallurgy is the process of isolating elements from their ores or compounds. Ores are naturally occurring minerals that contain metals and need to be concentrated to remove impurities. Concentration methods like froth flotation and magnetic separation are used for this purpose.
Extraction of metals from ores can be done through pyrometallurgy or hydrometallurgy. Pyrometallurgy involves high-temperature processes like smelting and roasting, while hydrometallurgy uses aqueous solutions for extraction. Electrolytic refining is used to obtain pure metals through electrolysis.
Specific extraction methods depend on the metal being extracted. Highly reactive metals like sodium and potassium are obtained through electrolysis of their molten salts. Less reactive metals like copper and silver are extracted through roasting and reduction. Gold and silver are extracted using the cyanide process. Aluminum is extracted by electrolysis of alumina, and zinc is obtained through roasting and reduction. Mercury extraction involves heating cinnabar, and titanium extraction involves reduction, distillation, and purification.
General Principles And Processes Of Isolation Of Elements Important Points , General Principles And Processes Of Isolation Of Elements Important Points , General Principles And Processes Of Isolation Of Elements Important Points , General Principles And Processes Of Isolation Of Elements Important Points

NCERT Chemistry Class 12 Chapter 6 – 25 General Principles And Processes Of Isolation Of Elements Important Points
There are 25 important points on general principles and processes of isolation of elements –
- The process of isolating elements from their ores or compounds is known as metallurgy.
- Metallurgy involves various steps like mining, concentration, extraction, and refining.
- Ores are naturally occurring minerals from which metals can be extracted profitably.
- The concentration of ores is done to remove impurities and increase the metal content.
- The two common methods of concentration are froth flotation and magnetic separation.
- Froth flotation is used to separate ores based on the difference in the wettability of minerals.
- Magnetic separation is used when one of the components of the ore is magnetic in nature.
- The extraction of metals from their ores can be done by either pyrometallurgy or hydrometallurgy.
- Pyrometallurgy involves the use of high temperatures to extract metals, usually through smelting or roasting.
- Hydrometallurgy involves the use of aqueous solutions, usually acids or bases, to extract metals.
- Electrolytic refining is used to obtain metals in their pure form by using electrolysis.
- The metal to be refined is made the anode, and a pure metal is used as the cathode in the electrolytic cell.
- During electrolysis, metal cations migrate to the cathode and get deposited in the pure form.
- The process of reducing a metal oxide using a reducing agent like carbon is called smelting.
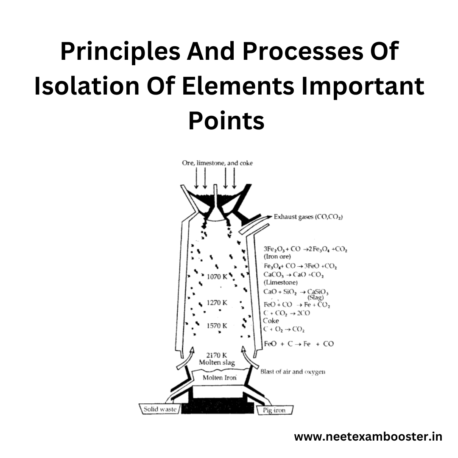
- The reduction of metal oxides by carbon is known as the process of extraction of metals in a blast furnace.
- The blast furnace is used for the extraction of iron and is operated at a high temperature.
- The extraction of highly reactive metals like sodium and potassium is done by electrolysis of their molten salts.
- The extraction of less reactive metals like copper and silver is done by roasting followed by reduction.
- Cyanide process is used for the extraction of gold and silver from their ores.
- The extraction of aluminum is done by the electrolysis of alumina dissolved in molten cryolite.
- The extraction of zinc is done by roasting the ore followed by reduction with carbon or carbon monoxide.
- The extraction of mercury is done by heating the ore, cinnabar, in the presence of air.
- The extraction of copper from its sulfide ores is done by the process of smelting followed by electrolytic refining.
- The extraction of silver from its ores is often done by the process of cyanidation.
- The extraction of titanium from its ores involves several steps including reduction, distillation, and purification.
Some Important Questions From Biology Class 11
| Chapter Name | Quiz Link |
| The Living World | Play Now |
| Biological Classification | Play Now |
| Plant Kingdom | Play Now |
| Animal Kingdom | Play Now |
| Morphology of flowering plants | Play Now |
| Anatomy of flowering plants | Play Now |
| Cell: the unit of life | Play Now |
| Biomolecules | Play Now |
| Cell Cycle and cell division | Play Now |
| Transport in Plants | Play Now |
| Structural organisation in Animals | Play Now |
| Mineral nutrition | Play Now |
| Photosynthesis in higher plants | Play Now |
| Respiration in plants | Play Now |
| Plant Growth and development | Play Now |
| Digestion and Absorption | Play Now |
| Breathing and Exchange of Gases | Play Now |
| Body fluids and circulation | Play Now |
| Excretory products and their elimination | Play Now |
| Locomotion and Movement | Play Now |
| Neural Control and Coordination | Play Now |
| Chemical Coordination and Integration | Play Now |
Some Important Questions From Biology Class 12
| Chapter Name | Quiz Link |
| Reproduction in organism | Play Now |
| Sexual reproduction in flowering plant | Play Now |
| Human reproduction | Play Now |
| Reproductive health | Play Now |
| Principles of inheritance and variation | Play Now |
| Molecular basis of inheritance | Play Now |
| Evolution | Play Now |
| Human health and disease | Play Now |
| Strategies for enhancement in food product | Play Now |
| Microbes in human welfare | Play Now |
| Biotechnology principles and processes | Play Now |
| Biotechnology and its application | Play Now |
| Organism and population | Play Now |
| Ecosystem | Play Now |
| Biodiversity and its conservation | Play Now |
| Environment issue | Play Now |




 155 out of 200 questions were directly asked from these notes in NEET 2024
155 out of 200 questions were directly asked from these notes in NEET 2024

Pingback: Define Linkage – Biology - NEET EXAM BOOSTER